- Thyroid
- A Multicenter, Randomized, Controlled Trial for Assessing the Usefulness of Suppressing Thyroid Stimulating Hormone Target Levels after Thyroid Lobectomy in Low to Intermediate Risk Thyroid Cancer Patients (MASTER): A Study Protocol
-
Eun Kyung Lee, Yea Eun Kang, Young Joo Park, Bon Seok Koo, Ki-Wook Chung, Eu Jeong Ku, Ho-Ryun Won, Won Sang Yoo, Eonju Jeon, Se Hyun Paek, Yong Sang Lee, Dong Mee Lim, Yong Joon Suh, Ha Kyoung Park, Hyo-Jeong Kim, Bo Hyun Kim, Mijin Kim, Sun Wook Kim, Ka Hee Yi, Sue K. Park, Eun-Jae Jung, June Young Choi, Ja Seong Bae, Joon Hwa Hong, Kee-Hyun Nam, Young Ki Lee, Hyeong Won Yu, Sujeong Go, Young Mi Kang, MASTER study group
-
Endocrinol Metab. 2021;36(3):574-581. Published online May 26, 2021
-
DOI: https://doi.org/10.3803/EnM.2020.943
-
-
6,293
View
-
268
Download
-
8
Web of Science
-
11
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
Postoperative thyroid stimulating hormone (TSH) suppression therapy is recommended for patients with intermediate- and high-risk differentiated thyroid cancer to prevent the recurrence of thyroid cancer. With the recent increase in small thyroid cancer cases, the extent of resection during surgery has generally decreased. Therefore, questions have been raised about the efficacy and long-term side effects of TSH suppression therapy in patients who have undergone a lobectomy.
Methods
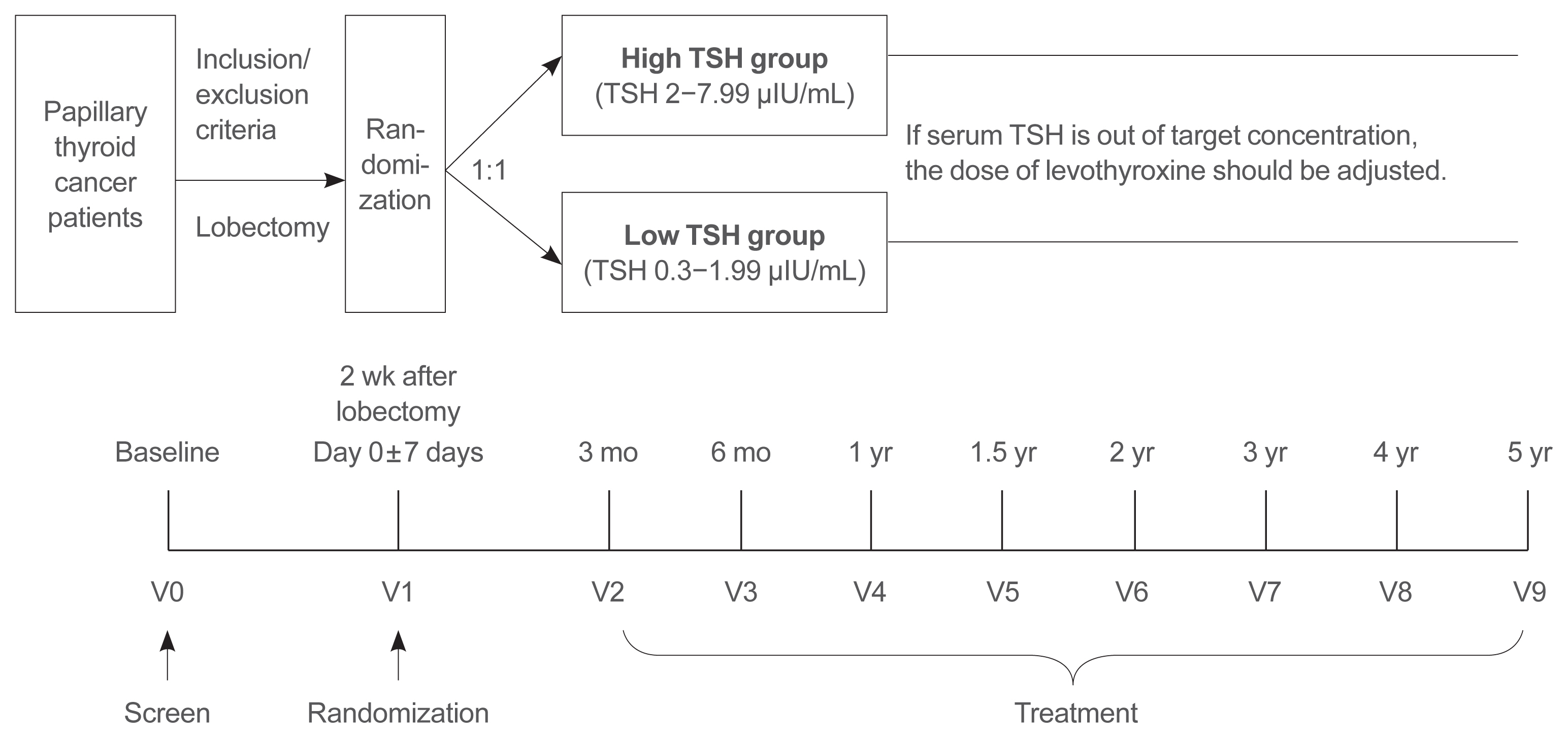
This is a multicenter, prospective, randomized, controlled clinical trial in which 2,986 patients with papillary thyroid cancer are randomized into a high-TSH group (intervention) and a low-TSH group (control) after having undergone a lobectomy. The principle of treatment includes a TSH-lowering regimen aimed at TSH levels between 0.3 and 1.99 μIU/mL in the low-TSH group. The high-TSH group targets TSH levels between 2.0 and 7.99 μIU/mL. The dose of levothyroxine will be adjusted at each visit to maintain the target TSH level. The primary outcome is recurrence-free survival, as assessed by neck ultrasound every 6 to 12 months. Secondary endpoints include disease-free survival, overall survival, success rate in reaching the TSH target range, the proportion of patients with major cardiovascular diseases or bone metabolic disease, the quality of life, and medical costs. The follow-up period is 5 years.
Conclusion
The results of this trial will contribute to establishing the optimal indication for TSH suppression therapy in low-risk papillary thyroid cancer patients by evaluating the benefit and harm of lowering TSH levels in terms of recurrence, metabolic complications, costs, and quality of life.
-
Citations
Citations to this article as recorded by  - Effect of thyroid-stimulating hormone suppression on quality of life in thyroid lobectomy patients: interim analysis of a multicenter, randomized controlled trial in low- to intermediate-risk thyroid cancer patients (MASTER study)
Ja Kyung Lee, Eu Jeong Ku, Su-jin Kim, Woochul Kim, Jae Won Cho, Kyong Yeun Jung, Hyeong Won Yu, Yea Eun Kang, Mijin Kim, Hee Kyung Kim, Junsun Ryu, June Young Choi
Annals of Surgical Treatment and Research.2024; 106(1): 19. CrossRef - Clinical impact of coexistent chronic lymphocytic thyroiditis on central lymph node metastasis in low- to intermediate-risk papillary thyroid carcinoma: The MASTER study
Da Beom Heo, Ho-Ryun Won, Kyung Tae, Yea Eun Kang, Eonju Jeon, Yong Bae Ji, Jae Won Chang, June Young Choi, Hyeong Won Yu, Eu Jeong Ku, Eun Kyung Lee, Mijin Kim, Jun-Ho Choe, Bon Seok Koo
Surgery.2024; 175(4): 1049. CrossRef - Dynamic Changes in Treatment Response af-ter 131I in Differentiated Thyroid Cancer and Their Relationship with Recurrence Risk Stratification and TNM Staging
璐 狄
Advances in Clinical Medicine.2024; 14(03): 1083. CrossRef - ASO Author Reflections: Active Surveillance may be Possible in Patients with T1b Papillary Thyroid Carcinoma Over 55 Years of Age Without High-Risk Features on Preoperative Examinations
Ho-Ryun Won, Eonju Jeon, Da Beom Heo, Jae Won Chang, Minho Shong, Je Ryong Kim, Hyemi Ko, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Kyong Hye Joung, Ji Min Kim, Younju Lee, Sung-Woo Kim, Young Ju Jeong, Yong Bae Ji, Kyung Tae, Bon Seok Koo
Annals of Surgical Oncology.2023; 30(4): 2254. CrossRef - Outcomes and Trends of Treatments in High‐Risk Differentiated Thyroid Cancer
Arash Abiri, Khodayar Goshtasbi, Sina J. Torabi, Edward C. Kuan, William B. Armstrong, Tjoson Tjoa, Yarah M. Haidar
Otolaryngology–Head and Neck Surgery.2023; 168(4): 745. CrossRef - Current Controversies in Low-Risk Differentiated Thyroid Cancer: Reducing Overtreatment in an Era of Overdiagnosis
Timothy M Ullmann, Maria Papaleontiou, Julie Ann Sosa
The Journal of Clinical Endocrinology & Metabolism.2023; 108(2): 271. CrossRef - Age-Dependent Clinicopathological Characteristics of Patients with T1b Papillary Thyroid Carcinoma: Implications for the Possibility of Active Surveillance
Ho-Ryun Won, Eonju Jeon, Da Beom Heo, Jae Won Chang, Minho Shong, Je Ryong Kim, Hyemi Ko, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Kyong Hye Joung, Ji Min Kim, Younju Lee, Sung-Woo Kim, Young Ju Jeong, Yong Bae Ji, Kyung Tae, Bon Seok Koo
Annals of Surgical Oncology.2023; 30(4): 2246. CrossRef - Potential impact of obesity on the aggressiveness of low- to intermediate-risk papillary thyroid carcinoma: results from a MASTER cohort study
Mijin Kim, Yae Eun Kang, Young Joo Park, Bon Seok Koo, Eu Jeong Ku, June Young Choi, Eun Kyung Lee, Bo Hyun Kim
Endocrine.2023; 82(1): 134. CrossRef - Differentiated thyroid cancer: a focus on post-operative thyroid hormone replacement and thyrotropin suppression therapy
Benjamin J. Gigliotti, Sina Jasim
Endocrine.2023; 83(2): 251. CrossRef - Thyroid stimulating hormone suppression and recurrence after thyroid lobectomy for papillary thyroid carcinoma
Mi Rye Bae, Sung Hoon Nam, Jong-Lyel Roh, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim
Endocrine.2022; 75(2): 487. CrossRef - The Concept of Economic Evaluation and Its Application in Thyroid Cancer Research
Kyungsik Kim, Mijin Kim, Woojin Lim, Bo Hyun Kim, Sue K. Park
Endocrinology and Metabolism.2021; 36(4): 725. CrossRef
- Insulin-dependent Stimulation of a Subtype of p38Map Kinases and Its Role in Insulin's Antiapoptotic Activity.
-
Shin Hae Kang, Ji Hoon Kang, Hee Kyoung Kang, Dae Ho Lee, Young Ki Lee, Deok Bae Park
-
J Korean Endocr Soc. 2004;19(4):358-368. Published online August 1, 2004
-
-
-
 Abstract Abstract
 PDF PDF
- BACKGROUND
The p38 mitogen-activated protein kinases (p38Map kinases) are a family of prolinedirected serine/threonine kinases. At least four isoforms of p38Map kinases have been identified; however, their physiological significances remain to be understood. Recently, the role of p38Map kinase in insulin-stimulated glucose uptake has been suggested. The present study aimed to investigate which isoform(s) were responsive to insulin stimulation. In addition, the activities of p38 Map kinase isoforms that may participate in the insulin's antiapoptotic function in CHO-IR cells were also determined. METHODS: Chinese hamster ovary cells, expressing wild- or mutated human insulin receptors (CHO-IR cells), were used to investigate whether insulin can stimulate any of the isoform(s) of the p38Map kinases. The p38Map kinase activity was determined by measuring the degree of 32P-labelling of ATF-2 protein, a specific substrate of p38Map kinase. A DNA laddering assay was performed to examine the degree of apoptosis and a RT-PCR analysis to determine which isoform(s) of the p38Map kinases were expressed in response to insulin. RESULTS: p38Map kinase activation by insulin was sharply suppressed in only the CHO-IR/A1018K cells, which lack the intrinsic tyrosine kinase activity of insulin receptors. Insulin stimulation of p38Map kinase was insensitive to SB203580, an inhibitor of the alpha(alpha)-and beta(beta)-isoforms of p38Map kinases. Moreover, orthovanadate, known as a specific stimulator of the gamma(gamma)-and delta(delta-) isoforms, stimulated the p38Map kinase activity in CHO-IR cells. Insulin increased the degree of mRNA expression of the delta-isoform, but not that of the alpha-isoform p38Map kinase. Interestingly, PD98059, an inhibitor of ERK, suppressed p38Map kinase stimulation, as well as the antiapoptotic protection of cells by insulin. As insulin was found to still protect ERK-lacking cells (CHO-IR/ SOS) from apoptosis, any substantial role(s) of ERK might be excluded. CONCLUSION: Our data suggest that insulin may stimulate the activity and expression of the-isoform of p38Map kinase in a MEK1/2-dependent manner. The involvement of the delta-isoform of p38Map kinase in insulin's antiapoptotic protection was also suggested, but remains to be investigated further to clarify the nature of its mechanism of action
|